Background: One of the most devastating complications of Hemophilia A or B (HemA, HemB) is end-stage joint damage characterized by chronic pain and functional disability. Joint bleeding is the best predictor of joint damage in hemophilia. A wide range of new therapeutics from extended half-life factor (EHL) concentrates to non-factor therapies such as factor VIII mimetics, hemostatic rebalancers and gene therapy have been recently approved or are in late-stage clinical trials that hold the promise of decreased joint disease in severe hemophilia.
Specific Aim: Given the progressive improvements in the therapeutic armamentarium of hemophilia care, this study aimed to determine current predictors of joint bleeding in individuals with severe hemophilia on continuous prophylaxis in the context of a national surveillance project.
Methods: Eligible participants were males with severe HemA or HemB, aged 2 to 44 years, on continuous prophylaxis therapy and participating in Community Counts (CC) during the period December 2013 to November 30, 2022. CC is a bleeding disorders surveillance project funded by the Centers for Disease Control and Prevention and administered through the American Thrombosis and Hemostasis Network in 141 federally funded Hemophilia Treatment Centers across the United States. CC collects data on sociodemographics, bleeding episodes, treatment products, and health outcomes during patients' annual comprehensive care visits. Data were analyzed using annualized joint bleeding rates (AJBR), calculated using the number and location of all joint bleeds occurring in 10 joints (shoulders, elbows, hips, knees, ankles) during the year since the last surveillance visit based on infusion logs and/or other patient records. Age and other demographic subgroups were compared via rate ratios (RR) and 95% confidence intervals (CI). Methods accounting for multiple measurements from the same individuals over time were used for multivariate analyses. AJBR differences according to demographic and clinical variables were assessed via p values comparing Beta coefficients from these models.
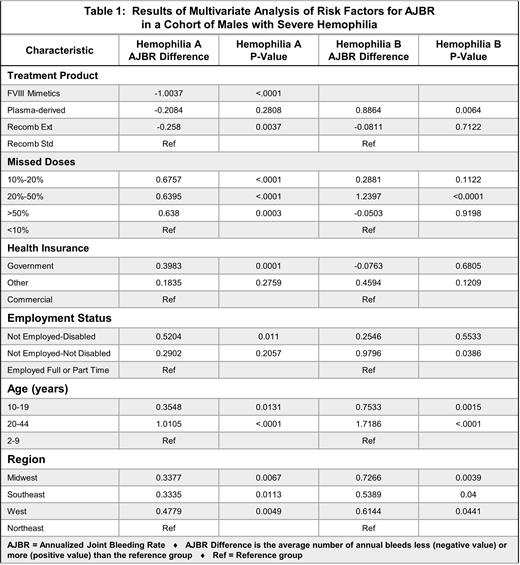
Results: The HemA cohort included 2505 persons, 8542 observations years and 13,224 joint bleeds for an overall AJBR of 1.55. The HemB cohort included 439 persons, 1488 observation years and 1326 joint bleeds for an overall AJBR of 0.89. Joint bleeding increased with age. In comparison to those ages 2-9 years, RRs were significantly increased for ages 10-19 and 20-44 for both HemA (1.23 [CI 1.1-1.3]) and 3.17 [CI 3-3.4], respectively), and HemB (2.2 [1.8-2.7] and 5.8 [4.8-7], respectively). Uninsured persons had higher AJBR than insured (HemA, RR 1.8 [1.6-2.1] and HemB, RR 6.9 [5.2-9.]. Table 1 compares characteristics associated with AJBR in multivariate analysis. The use of the FVIII mimetic in persons with HemA was associated with fewer joint bleeds (-1.00 joint bleeds per year). Persons with HemA showed modest decreases in AJBR with EHL therapies (-0.26), a finding not observed in persons with HemB. Worse AJBR outcomes were associated with missed doses for persons with both HemA and HemB. Joint bleeding was significantly associated with unemployment with disability for persons with HemA but not HemB. Trends in AJBR were similar between HemA and HemB for the following: adults had more joint bleeding than children, including adolescents aged 10-19 who are often engaged in team sports; compared with the Northeast, AJBR were higher in all other geographic regions; persons on Government insurance had higher AJBR than persons on Commercial insurance.
Discussion: In a multivariate analysis of data on a large longitudinal cohort of persons with severe hemophilia on prophylaxis, use of the FVIII mimetic, ≥90% treatment adherence and treatment in the Northeast United States were associated with significantly lower AJBR. The increased joint bleeding rate in adults compared with children and adolescents may be related to pre-existing joint disease, less intense prophylactic regimens or decreased activity and fitness. Additional studies can further inform optimal hemophilia therapy regimens.
Disclosures
Manco-Johnson:Novo: Honoraria; Bayer: Honoraria; Spark: Honoraria; CSL Behring: Honoraria; Genentech: Honoraria. Acharya:Bayer: Honoraria; Pfizer: Honoraria. Ahuja:State of Ohio Rare Disease Advisory Council: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; TraumaChek: Patents & Royalties; Genentech: Honoraria; NovoNordisk: Honoraria; CSLBehring: Honoraria; XaTec Inc: Research Funding; ClotChip: Patents & Royalties. Chitlur:Novo Nordisk: Consultancy, Honoraria; Children's Foundation: Research Funding; HRSA/MCHB: Research Funding; BPL Inc: Honoraria; Takeda: Honoraria; Genentech Inc: Honoraria, Research Funding; Genzyme Corp: Honoraria; Agios Pharmaceuticals: Honoraria, Research Funding; Novartis Pharmaceuticals: Research Funding. Sharathkumar:Amgen: Research Funding; Genentech: Consultancy; Spark Therapeutics: Consultancy.